Abstract
Background: Despite advances, most patients with multiple myeloma (MM) eventually relapse and progress through multiple lines of therapy. To complement clinical trial data, real-world evidence on treatment patterns can provide valuable insights on clinical practice in different countries and help identify and address unmet medical needs. Here, we report real-world (RW) treatment use in patients from European data sets who initiated third-line (3L) treatment for relapsed or refractory (RR) MM.
Methods: This retrospective, noninterventional analysis used claims data from the German AOK PLUS health insurance fund and Italian local health units (2012-2020) and applied an algorithm based on prescription and procedure codes. The algorithm defined the start of a new line of therapy as the introduction of a new agent not part of the prior line. Agents given within 30 days of start defined the regimen. Retreatment after >6 months of discontinuation were also considered a new line. Patients initiating 3L treatment from 2016 to 2020 (index date range) were identified. Baseline characteristics and treatment patterns were then reported.
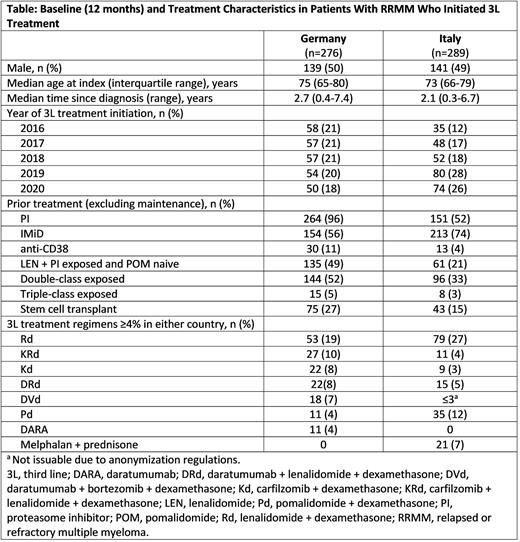
Results: Overall, 276 patients from Germany and 289 patients from Italy who met selection criteria were identified (Table). Patients in Germany and Italy had similar baseline characteristics in terms of sex, age, and time since diagnosis; 27% and 15% of patients, respectively, had prior stem cell transplant. Prior to 3L treatment, IMiD use was lower (56% vs 74%) and proteasome inhibitor (PI) use was higher (96% vs 52%) among patients in Germany vs those in Italy, respectively. At 3L, the proportion of double class-exposed patients in Germany was higher vs Italy (52% vs 33%).
In Germany, common first-line (1L) regimens were bortezomib (BORT) + dexamethasone (DEX; Vd) (46%), melphalan (MEL) + prednisone (PRED) + BORT (14%), and Vd + cyclophosphamide (8%). The most common 2L treatment regimens were lenalidomide (LEN) + DEX (Rd; 30%), Vd (11%), and carfilzomib (CFZ) + Rd (8%). By 3L, 95% and 54% of patients had received prior BORT- and LEN-based regimens, respectively. In 3L, combinations were often based on LEN and/or CFZ with daratumumab (DARA) monotherapy and pomalidomide (POM) + DEX (Pd) also contributing about 4% each (Table).
In Italy, common 1L regimens included MEL ± DEX ± PRED (22%), Vd (6%), and thalidomide (THAL) + DEX (5%). Rd (40%) and Vd (8%) were the most common 2L regimens in Italy; by 3L, 56% and 49% of patients had received prior LEN- and BORT-containing regimens, respectively.
Overall, Pd was the second most common 3L treatment in Italy and its use was higher than in Germany (12% vs 4%), whereas 3L use was lower for regimens based on CFZ (10% vs 21%) and DARA (9% vs 29%). Patients in Italy also had higher 3L use of conventional therapies such as MEL compared with patients in Germany (15% vs 3%).
Retreatment patterns in Germany and Italy showed that 60% and 82% of patients initiating 3L treatment, respectively, had prior exposure to the same agent class (IMiD, PI, monoclonal antibodies). Of 50 patients in Germany with BORT treatment in 3L, 47 (94%) also had prior BORT; of 135 patients with LEN treatment in 3L, 47 (35%) had prior LEN. Of 40 patients in Italy with BORT treatment in 3L, 31 (78%) had prior BORT; of 131 patients with LEN treatment in 3L, 82 (63%) had prior LEN. Additionally, of 13 patients in Italy that received prior THAL, all were retreated with THAL in 3L.
Conclusions: This study of European claims datasets provides a perspective of RW clinical practice with a focus on patients initiating 3L treatment from 2016 to 2020 for comparison with the evolving treatment landscape for RRMM. LEN-based regimens were most commonly observed in 3L, with high proportions of CFZ- and POM-based regimens in Germany and Italy, respectively. This finding is likely the result of BORT- and LEN-based regimens being common in 1L and 2L, respectively, reflecting what was approved and/or used before and during 2016. Many 3L patients were retreated with the same agent class used in a prior line. BORT use in 3L was prominent for retreatment in Germany and Italy, which may reflect BORT's prior use in a fixed-duration regimen. Notably, retreatment with LEN in 3L was also prominent in Italy. Comparisons with more recent index groups could help elucidate treatment evolution over time. Additionally, it would be relevant to understand how European RW clinical practice compares with observations in the US setting.
Disclosures
Lehne:Cytel Inc.: Other: Employee. Zamagni:janssen, bms, amgen, roche, pfizer, sanofi, gsk: Honoraria, Other. Zhuleku:Cytel Inc.: Other: Employee. Ghiani:IPAM e.V.: Other: Staff members. Hanna:GSK: Current Employment, Current equity holder in publicly-traded company. Maywald:AOK PLUS: Other: Employee. Wilke:IPAM e.V.: Other: Staff members; GSK, Novo Nordisk, Abbvie, Merck, BMS, LEOPharma, Bayer, Boehringer Ingelheim: Honoraria. Perera:GSK: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal